Ultimate Guide to pH vs Alkalinity and a Sparkling Pool
As an Amazon Associate, I earn from qualifying purchases.
Table of Contents
Understanding pH vs Alkalinity in Swimming Pools
Pool water chemistry can seem complex for new (and even some old) pool owners. The topic of pH vs alkalinity is possibly among the more challenging aspects.

Along with pool sanitation, the relationship between pH and alkalinity is the backbone of pool water chemistry. They influence water clarity, swimmer’s health & safety, and the lifespan of pool equipment.
In a nutshell, pH refers to how acidic or basic your pool water is, while alkalinity is a measure of the water’s ability to resist changes in pH.
Of course, you need more than this information to develop any meaningful understanding of pH vs alkalinity in a swimming pool.
Do not worry, for in this article I am going to break down the difference between pH and alkalinity, their ideal levels, their impact on your pool, and how to manage them.
Key Takeaways
- Keep pH between 7.2 and 7.8 (ideally between 7.4 and 7.6) for balanced water.
- Maintain total alkalinity at 80–120 ppm to stabilize pH.
- Test pool water weekly to monitor pH and alkalinity.
- Use sodium bicarbonate to raise alkalinity effectively.
- Add muriatic acid to lower pH and alkalinity safely.
- Avoid pH bounce by keeping alkalinity in range.
- Regular balance prevents scaling, corrosion, and swimmer discomfort.
Definitions of pH and Alkalinity
Before diving into the details of pool chemistry, it’s important to understand what pH and alkalinity mean and how they differ.
What is pH?
pH is a measure of how acidic or basic water is, using a scale from 0 to 14:
- A pH of 7 is neutral.
- Values below 7 indicate acidic water.
- Values above 7 indicate basic water.
If the pH is too low (acidic), it can damage pool surfaces and equipment. If it’s too high (basic), it can cause scaling and reduce the effectiveness of sanitizers, like chlorine.
What is Alkalinity?
Alkalinity, often referred to as Total Alkalinity (TA), measures the water’s ability to resist changes in pH. Think of it as a pH buffer. Proper alkalinity levels stabilize pH and prevent sudden swings that can disrupt the pool’s water chemistry.
Alkalinity is measured in parts per million (ppm).
The Difference Between pH and Alkalinity
While pH tells you if the water is acidic, neutral, or basic, alkalinity indicates how well the water can maintain its pH level. They are closely related but serve different purposes:
- pH directly impacts swimmer comfort and pool health.
- Alkalinity ensures the pH stays stable.
By balancing both, you can prevent common issues like acidic water, scaling, or erratic pH changes.
Role of pH in Pool Water Chemistry
The pH level affects a lot of things in pool water chemistry, from the chlorine effectiveness to the comfort of swimmers and the longevity of the pool’s equipment and surfaces.
A. Effect on Chlorine Effectiveness
For your pool sanitizer, typically chlorine, to work effectively, the pH must be within the recommended range. Here is what happens when pH is out of the recommended range:
- Low pH (below 7.2): Chlorine becomes too aggressive, which can lead to over-sanitization and cause irritation to swimmers. Chlorine consumption is high too!
- High pH (above 7.8): Chlorine loses its effectiveness, allowing algae and bacteria to thrive.
The sweet spot for chlorine is when the pH is between 7.2 and 7.8 (ideally between 7.4 and 7.6). This ensures that your pool water stays clean and safe.
B. Effect on Swimmer Comfort
Swimmer comfort depends heavily on balanced pH levels.
- Low pH can cause burning eyes, itchy skin, and even irritation to nasal passages.
- High pH often leads to dry skin and a feeling of heaviness in the water.
When the pH is balanced, swimming feels natural and refreshing without causing discomfort.
C. Effect on Pool Surfaces (Etching and Scaling)
The pH of your pool water directly impacts the physical condition of your pool.
- Low pH (acidic water): This can corrode metal fixtures, degrade pool liners, and cause etching on plaster surfaces.
- High pH (basic water): This promotes the formation of calcium scale, which appears as white, crusty deposits on tiles and pool walls.
Maintaining a balanced pH protects your investment by keeping your pool surfaces and equipment in good condition.
Role of Alkalinity in Pool Water Chemistry
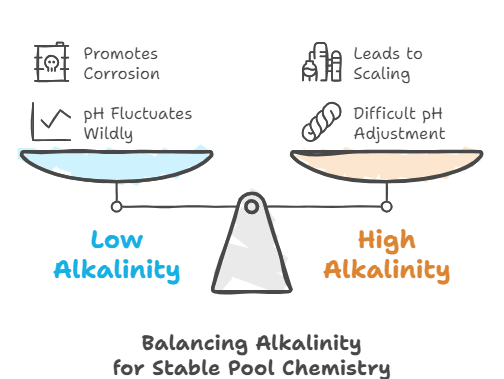
While pH measures whether the pool water is acidic or basic, alkalinity determines how stable that pH level remains. It acts as a pH buffer, preventing drastic fluctuations that can disrupt pool water chemistry.
Alkalinity as a pH Buffer
When you add chemicals, contaminants, or even fresh water to your pool, the pH may shift. The correct level of Total Alkalinity (TA) absorbs these changes, stabilizing the pH and keeping the water chemistry in balance.
For example:
- If alkalinity is too low, the pH can fluctuate wildly, creating a condition called “pH bounce.”
- If alkalinity is too high, it can make adjusting pH difficult and lead to scaling issues.
Alkalinity and LSI (Langelier Saturation Index)
The Langelier Saturation Index (LSI) is a measure of water balance that considers factors like pH, alkalinity, calcium hardness, and water temperature.
- High alkalinity contributes to a positive LSI, increasing the likelihood of calcium scale.
- Low alkalinity can lead to a negative LSI, promoting corrosion and etching.
By keeping alkalinity within its recommended range, you can maintain overall water balance and prevent problems with scaling or corrosive water.
Impact on Pool Equipment
Maintaining Total Alkalinity (TA) within the recommended range helps protect your pool’s equipment, such as pumps, heaters, and filters.
Low alkalinity can lead to corrosive water that damages metal parts, while high alkalinity encourages scaling that clogs equipment.
Ideal Ranges for pH and Alkalinity
Keeping your pool water in balance starts with maintaining the right levels of pH and total alkalinity. Staying within these ranges ensures your pool is safe, comfortable, and properly sanitized.
Ideal pH Range
The recommended pH range for pool water is 7.2 to 7.8 (ideally between 7.4 and 7.6).
- At this level, the water is neither too acidic nor too basic.
- pH in this range optimizes chlorine effectiveness, protects surfaces and equipment, and ensures swimmer comfort.
Ideal Alkalinity Range
The recommended range for total alkalinity is 80 to 120 ppm (parts per million).
- Levels below 80 ppm can cause pH bounce, making the water unstable and difficult to maintain.
- Levels above 120 ppm may result in scaling and cloudy water, as well as hinder pH adjustments.
Why Balance Matters
- When both pH and alkalinity are within their ideal ranges, the pool water remains stable and well-balanced.
- Imbalanced levels lead to problems like acidic water, scaling, or inefficient use of chlorine.
Relationship Between pH and Alkalinity
pH and alkalinity are closely connected in pool chemistry. While they are distinct measures, changes to one can significantly impact the other.
How Alkalinity Affects pH Stability
Total Alkalinity (TA) acts as a pH buffer, helping to stabilize the water’s acidity or basicity.
- If alkalinity is too low, the pH can fluctuate easily due to chemical additions, rain, or contaminants, creating a situation known as a “pH bounce.”
- Proper alkalinity levels keep the pH steady, ensuring the need for fewer adjustments and a more consistent pool environment.
For example:
- Adding a small amount of acid to a pool with balanced alkalinity will only slightly lower the pH.
- In contrast, the same amount of acid in a pool with low alkalinity will cause a sharp drop in pH.
How pH Adjustments Impact Alkalinity
Raising or lowering the pH also influences total alkalinity:
- Raising pH with soda ash or similar chemicals can also increase alkalinity.
- Lowering pH with muriatic acid or dry acid will reduce alkalinity as well.
This interplay means adjustments need to be made carefully to avoid throwing both out of balance.
Challenges in Balancing Both
Because of their interconnectedness, adjusting one without considering the other can create challenges:
- Raising alkalinity with sodium bicarbonate often increases the pH slightly.
- Lowering pH with acid can reduce alkalinity. As such you will need to raise alkalinity by using baking soda (sodium bicarbonate).
The key is to adjust gradually and test frequently. Using tools like a pool calculator can help you make precise changes without disrupting the balance.
Consequences of Imbalanced pH and Alkalinity
You can expect a range of problems when pH and alkalinity are not within their ideal ranges. They concern, swimmer comfort & safety, pool equipment life, and water quality.
Low pH and Alkalinity (Acidic Water)
If the pH drops below 7.2 and alkalinity is below 80 ppm, the water becomes too acidic, causing:
- Corrosion: Acidic water can corrode metal components like ladders, heaters, and pump parts.
- Etching: Plaster and concrete surfaces may erode, weakening the pool structure over time.
- Irritation: Swimmers may experience burning eyes, itchy skin, and discomfort due to the aggressive nature of acidic water.
- Chlorine Over-Effectiveness: While chlorine works well in low pH, it becomes overly aggressive, potentially causing unnecessary chemical exposure and increased cost of pool sanitization.
High pH and Alkalinity (Basic Water)
When pH rises above 7.8 and alkalinity exceeds 120 ppm, the water becomes too basic, resulting in:
- Calcium Scaling: White, chalky deposits form on pool surfaces, tiles, and equipment. The result is a drop in efficiency of the filtration system. Cleaning of pool surfaces becomes difficult and the pool looks jaded.
- Cloudy Water: Excessive alkalinity can cause precipitation of calcium and other minerals, reducing water clarity.
- Reduced Chlorine Effectiveness: High pH hinders chlorine’s ability to sanitize, promoting algae and bacteria to grow.
- Swimmer Discomfort: Basic water can cause dry, itchy skin and hair.
pH Bounce from Low Alkalinity
If total alkalinity is too low, even small changes—such as rainfall or chemical additions—can cause the pH to fluctuate erratically. This instability makes it difficult to maintain a balanced pool chemistry.
Scaling and Staining from High Alkalinity
Excessively high alkalinity can make it challenging to lower pH and often leads to calcium buildup on pool surfaces. Over time, this scaling can trap dirt and minerals, causing unsightly stains that are hard to remove.
Impact on Pool Maintenance
Imbalanced pH and alkalinity require more frequent chemical adjustments and cleaning. This not only increases maintenance time but also adds to pool care costs.
Basic Steps for Raising pH and Alkalinity
If your pool’s pH or total alkalinity drops below the ideal ranges, you’ll need to raise them to restore balance. Here’s how you can do it effectively.
How to Raise pH
Low pH (below 7.2) indicates that the water is too acidic. Here’s what you can do:
- Use Soda Ash (Sodium Carbonate):
- Add soda ash to the pool water. This is the most common method for increasing pH quickly.
- Follow the product’s instructions for dosage based on your pool’s size and current pH level.
- Add the chemical gradually while the pump is running to circulate the water.
- Aerate the Water (Optional):
- If your alkalinity is already balanced, aerating the water (e.g., running water features) can help raise the pH without affecting alkalinity.
- Retest the Water:
- Wait a few hours and retest the pH. Adjust as needed until it is within the recommended range of 7.2 to 7.8.
How to Raise Total Alkalinity
Low alkalinity (below 80 ppm) makes the water prone to pH bounce. Here’s how to fix it:
- Add Sodium Bicarbonate (Baking Soda):
- Use sodium bicarbonate, a safe and effective way to raise alkalinity.
- For every 10 ppm increase in alkalinity, add about 1.5 pounds of baking soda per 10,000 gallons of water.
- You can also use my Pool Baking Soda Calculator to determine the dosage for your pool.
- Spread the baking soda across the surface of the pool and allow the pump to circulate it.
- Adjust Gradually:
- Avoid adding too much at once. Test the water after 6–12 hours to measure the impact before adding more.
- Monitor the pH:
- Raising alkalinity may slightly increase pH, so be prepared to make minor pH adjustments, if needed.
Arm & Hammer Pure Baking Soda
America’s #1 Baking Soda Brand. Raises the total alkalinity level to ensure healthy swimming pool water. Helps prolong the life of pool surfaces and equipment.
Pro Tips for Adjusting pH and Alkalinity
- Always test the water before and after making adjustments using a reliable pool testing kit.
- Adjust Alkalinity before attempting pH adjustment to avoid “pH bounce.”
- Make changes gradually to avoid overcorrecting and throwing your pool chemistry out of balance.
- Run the pool pump during and after chemical additions to ensure even distribution.
Basic Steps for Lowering pH and Alkalinity
When your pool’s pH or total alkalinity is too high, it can lead to scaling, cloudy water, and ineffective sanitization. Here’s how to lower these levels safely and effectively.
How to Lower pH
High pH (above 7.8) indicates that the water is too basic. Follow these steps to bring it back to the ideal range:
- Use Muriatic Acid or Dry Acid:
- Muriatic acid is the most common chemical for lowering pH.
- Dry acid (sodium bisulfate) is an alternative that’s easier to handle.
- Add Acid Gradually:
- Dilute the acid in a bucket of water (if directed by the product instructions).
- Slowly pour the diluted acid around the edges of the pool while the pump is running.
- Wait and Retest:
- Allow the pool water to circulate for at least 4–6 hours before retesting the pH.
- Continue small adjustments until the pH falls between 7.2 and 7.8.
Recommended Muriatic Acid
Acid Blue Muriatic Acid by CPDI
Vapor Reduction Technology reduces up to 90% of harmful vapors compared to standard muriatic acid.
How to Lower Total Alkalinity
High alkalinity (above 120 ppm) makes it difficult to adjust pH and can lead to scaling. Here’s how to reduce it:
- Use Muriatic Acid:
- This is the most effective way to lower total alkalinity.
- Add the acid slowly in one spot of the pool to create a localized effect, known as “pool slugging.”
- Follow Acid Demand Tables:
- Use a chart or pool calculator to determine the correct amount of acid based on your pool size and alkalinity level.
- Adjust Gradually:
- Lowering alkalinity too quickly can cause the pH to drop drastically. Add the acid in small increments, allowing the water to circulate for several hours between doses.
- Aerate to Restore pH (Optional):
- After lowering alkalinity, your pH may dip. You can raise it back up naturally by aerating the water (e.g., using jets or running a fountain).
Pro Tips for Lowering pH and Alkalinity
- Always add acid to water and not the other way round, when diluting.
- Always add acid in small doses to avoid overcorrecting.
- Wear protective gear (gloves, goggles) when handling chemicals, especially muriatic acid.
- Never mix chemicals, and store them safely away from heat and moisture.
Frequently Asked Questions (FAQs)
What’s more important, pH or alkalinity?
Both are important for a balanced pool water chemistry, but alkalinity takes priority because it stabilizes pH, preventing erratic changes.
Do you adjust pH or alkalinity first?
Always adjust alkalinity first. Proper alkalinity stabilizes pH, making it easier to balance.
Does high pH mean high alkalinity?
Not necessarily. pH and alkalinity are related but can be high or low independently, depending on the chemical balance.
How can I raise pH but not alkalinity?
Use aeration, such as running water features or jets, to raise pH without affecting alkalinity.
Can rain affect my pool’s pH and alkalinity?
Yes, rain can lower both pH and alkalinity, especially if it’s acidic. Use a pool cover during the rainy season. Test and adjust after heavy rainfall.








