Importance of pH Buffer For Pools: Explained by an Expert
As an Amazon Associate, I earn from qualifying purchases.
Table of Contents
What Is A pH Buffer For Pools?
Balanced pool chemistry is extremely important for pool chemicals, especially the sanitizer, to work optimally. With so many pool chemicals and other uncontrollable external factors involved, this is easier said than done. How do you keep chlorine, pH & alkalinity within the recommended levels? pH buffer for pools. But what is a pH buffer for pools and why do you need it?
A pH buffer for pools is Sodium Bicarbonate (NaHCO3), which you may know as Baking Soda. For Chlorine to be effective, pool pH should remain within 7.4 and 7.6. But pH can fluctuate wildly, when different pool chemicals are added. Alkalinity from Baking Soda buffers the pH from fluctuations.
If the alkalinity is low, then the pH of your pool can fluctuate wildly, with the addition of any pool chemical. This is called the pH bounce. Fluctuating pool pH is not good for the swimmers and not good for the chlorine pool sanitizer.

By adding Baking Soda (Sodium Bicarbonate), which has a pH of 8, you increase both the pH and alkalinity of the pool. The increased alkalinity will buffer the pool from wild pH fluctuations.
Why Is pH Buffer For Pools Important?
When you talk of adding chlorine to pool water as a sanitizer or disinfectant, you are of course using the word “chlorine” in an easy to understand, day to day language.
In the language of chemistry, you are actually adding a compound of chlorine, such as Sodium Hypochlorite or Calcium Hypochlorite. They are salts of Hypochlorous Acid.
Sodium Hypochlorite is a salt of Sodium with the chemical formula NaOCl and Calcium Hypochlorite is a salt of Calcium with the chemical formula Ca(OCl)2.
Free Active Chlorine (FAC)
When they are mixed with pool water they release Hypochlorous Acid as per the equations below:
NaOCl + H2O => NaOH + HOCL
Ca(OCl)2 + 2H2O => 2CaOH + 2HOCL
The Hypochlorous Acid (HOCL) is what kills the germs, bacteria & algae. HOCL is Free Active Chlorine (FAC).
HOCL is, in fact, a weak acid. It kills the pathogens & algae by breaking down the enzymes of the cell walls and making the cell vulnerable.
HOCl co-exists in the water along with H+ and OCl- ions as per the equation below:
HOCL ⇔ H+ + OCl-
The measurement of Free Chlorine (FC) gives us the combined total of HOCl and OCl- ions. However, it is the HOCl, known as Free Active Chlorine (FAC) that is active in disinfection. You should be more concerned about the HOCl or the Free Active Chlorine in your pool.
pH & Free Chlorine Relationship
The amount of HOCL in the water is a function of the pH of the water as per the table below:
| pH | % HOCL | %OCl- |
| 5.00 | 99.71 | 0.29 |
| 5.50 | 99.09 | 0.91 |
| 6.00 | 97.18 | 2.82 |
| 6.50 | 91.50 | 8.50 |
| 7.00 | 77.53 | 22.47 |
| 7.50 | 49.00 | 51.00 |
| 8.00 | 25.65 | 74.35 |
| 8.50 | 11.00 | 89.00 |
| 9.00 | 3.34 | 96.66 |
| 9.50 | 1.00 | 99.00 |
| 10.00 | 0.34 | 99.66 |
As you can see, HOCl drops rather sharply as pH rises above 6.0. It would be great, from the disinfection point of view if the pH of the pool was kept at 6.0. This can’t be done as the pool water is too corrosive at a pH of 6.0.
So a pH of 7.5 (also the pH of the human eye) is the sweet spot. Low enough to have Free Available Chlorine (FAC) at around 50% and high enough to be comfortable for swimming.
A pH buffer for pools (such as Baking Soda) helps stabilize the pH level so that it stays close to the desired level of 7.5. You get the most bang for your buck out of the chlorine that you add to your pool.
Does Alkalinity Stabilize pH?
Alkalinity and pH are closely related but they are not the same thing, especially when it comes to pool chemistry.
pH is a measure of the acidity or alkalinity of water on a logarithmic scale with a range of 1-14.. The pH of distilled water is 7.0, that is in the middle of the scale. The lower the pH the more acidic the water is. The higher the pH the more alkaline the water is.
Note that the scale is logarithmic. So a lake is healthy at a pH of 6.5 but rainbow trout start to die at a pH of 6.0.
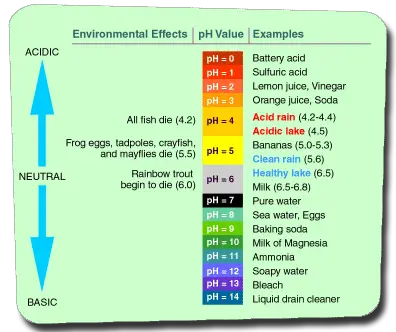
Alkalinity on the other hand is the quantity of alkaline substances, such as bicarbonate, carbonate, and hydroxide dissolved in the pool water. No wonder it is measured in ppm. You should keep the alkalinity of your pool between 80-120 ppm.
The alkaline substances can both attract and release Hydrogen ions. In other words they can interfere with Hydrogen and buffer against changes in pH.
Does Baking Soda Stabilize pH?
Baking Soda is useful if you want to increase the alkalinity of your pool without any noticeable increase in the pool pH. Soda Ash on the other hand will increase both alkalinity and pH. The table below is self explanatory.
| 10,000 gallon pool | |||
| Pool Chemical | Quantity Added | Increase in pH | Increase in Alkalinity |
| Baking Soda | 24 ounces | – | 10 ppm |
| Soda Ash | 12 ounces | 0.4 | 10 ppm |
So, yes, Baking Soda is an excellent pH buffer for pools.
Recommended Swimming Pool Chemicals
Best Baking Soda for Pools
Arm & Hammer is clearly the leader in Baking Soda. The product has multiple uses in the home, cleaning, laundry & deodorizing. It works great for increasing swimming pool alkalinity too! Order from Amazon using the link below:
2 x 13.5 Pounds Arm & Hammer Pure Baking Soda (27 Pounds Total)
Best Liquid CYA Free Pool Shock
Champion Liquid Chlorine (Sodium Hypochlorite) Pool Shock (12.5%) is easy-to-use, fast, and effective. This Pool Shock does not have Cyanuric Acid (CYA-stabilizer) and will not result in build-up of scale. Order from Amazon using the link below:
Liquid Chlorine Pool Shock (Case – 4 Gallons) – 12.5% Sodium Hypochlorite
Best Cal-Hypo Pool Shock
Calcium Hypochlorite Cal-Chlor chlorine granules, from In The Swim, provide 68% available chlorine for hard-hitting immediate results! Non-stabilized formula is ideal for super shocking or everyday use as it does not contain any Cyanuric Acid (CYA). Order from Amazon using the link below:
In The Swim Calcium Hypochlorite Chlorine Granular Pool Shock
Thank you very much for reading the post. I do hope you found it informative and helpful.






